On October 6, the Food and Drug Administration (FDA) released the long-awaited revisions to its draft guidance governing compounding by hospital and health-system pharmacies. The revised draft guidance deviates substantially from the prior version. FDA has removed the "one-mile radius” provision, which limited hospitals and health systems to sending compounded medications to clinical sites within one-mile of the compounding pharmacy. Instead, FDA is now proposing to institute a two-part risk-based enforcement approach for anticipatory non-patient-specific compounding by 503A hospital and health-system pharmacies. This guidance does not apply to those hospital/health-system pharmacies that are registered with FDA as 503B outsourcing facilities.
I. Proposed Compounding Enforcement Framework
FDA’s revised draft guidance is focused on two elements of compounding enforcement: the prescription requirement (i.e., the receipt of a valid prescription for an identified patient in advance of compounding) and the compounding of copies of commercially-available products. FDA strongly urges hospitals and health systems to either use 503B outsourcing facilities for non-patient-specific compounded medications whenever possible or to register their own facilities under 503B.
All 503A pharmacies are expected to compound almost exclusively for individually identified patients. However, in the revised draft guidance, FDA recognizes that hospital and health system clinical practice creates different demands for compounding, and provides some flexibility for hospitals to compound non-patient specific medications. In general, compounding enforcement for 503A pharmacies, including hospital and health-system pharmacies, will remain with state Boards of Pharmacy. FDA’s enforcement framework is case-by-case, allowing the agency significant discretion as to whether or not to intervene in enforcement.
There are two parts to FDA’s proposed hospital and health-system compounding framework: 1) a three-pronged safe harbor, and 2) for those who don’t meet the safe harbor requirements, additional risk factors that FDA will consider when determining whether to take enforcement action. It is critical to note that FDA’s enforcement approach is focused on non-patient-specific compounded medications. In practice, this means a drug that is compounded in advance of the receipt of a prescription for an individually-identified patient. The limitation on compounding does not apply to medications compounded after receipt of a prescription or order for an individually-identified patient. FDA’s enforcement framework also applies only to medications compounded under FDA’s definition of compounding – “the process of combining, mixing, or altering ingredients to create a medication tailored to the needs of an individual patient” – which is narrower than the USP definition of the term. Thus, FDA’s guidance applies only where a new drug is created by making changes to an existing product or mixing products together – it does not apply to preparation in accordance with package inserts or to general manipulation of a sterile product.

II. Initial ASHP Questions Regarding the Proposed Enforcement Framework
The revised guidance is currently in draft form, so ASHP and its members will have the opportunity to submit comprehensive comments before the document is finalized. ASHP is pleased that FDA removed the “one-mile radius” requirement from the guidance in response to our comments on the draft, but the 24-hour limitation on medication usage raises some questions. ASHP advocated for a time-based approach to ensuring safe compounding in hospital/health-system pharmacy, but we suggested FDA adopt the USP beyond-use dating (BUD) requirements. We remain concerned that the 24-hour limit introduces a new arbitrary time limitation rather than implementing BUD, a standard time-based metric that hospitals and health systems already observe.
ASHP will be seeking member feedback regarding how the 24-hour limitation might impact current practices and whether USP BUD or another metric(s) might be more practical. We also invite feedback on whether, and to what extent, it is practicable for hospitals and health systems to use 503B outsourcing facilities for non-patient-specific compounded medications. Specifically, given current system capacity and the types of compounded medications that would need to be purchased, could 503Bs meet demand?
III. Proposed Changes to “Essentially a Copy” Guidance for Hospitals and Health Systems
In the revised guidance, FDA also proposes to make some updates to its “Essentially a Copy” guidance (the “Copies Guidance”) to reflect the unique needs of hospitals and health systems. Under the current 503A Copies Guidance, a prescriber must document that a drug that is essentially a copy of a commercially-available product (i.e., it doesn’t differ enough in dosage form, strength, etc.) creates a significant clinical difference for a patient. In the revised draft guidance, FDA proposes to provide some flexibility for prescriber documentation of significant differences, particularly for medications that will need to be compounded regularly for an entire class of patient (e.g., pediatrics). Under the revised guidance, FDA will not take enforcement action against a hospital or health system for compounding copies of a commercially-available products regularly or inordinate amounts if the hospital and health system:
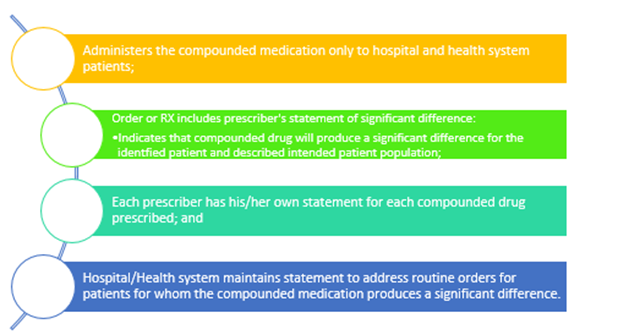
IV. Initial ASHP Questions Regarding the Copies Policy
ASHP’s understanding is that the proposed copies policy reflects ASHP’s request in comments on the draft guidance that hospitals and health systems be allowed to create a formulary-level or EHR means to document significant difference for commercially-available medications that must always be compounded for certain patient populations (e.g., pediatrics, cardiac). While we are appreciative of the agency’s incorporation of our members’ feedback in the revised guidance, the revised guidance is not prescriptive in how or where this documentation is maintained. The agency likely used less prescriptive language to ensure that hospitals and health systems had maximum flexibility in implementing the documentation requirement. Nevertheless, ASHP will be seeking member feedback regarding the proposed copies framework - specifically whether hospitals and health systems require additional detail from the agency to ensure they are documenting compounding of copies appropriately.
Next Steps
ASHP is currently developing a comprehensive webinar to walk through the differences between the initial draft guidance and the new revised guidance. We plan to include a question-and-answer component in that webinar to ensure members get the information they need and to facilitate information-sharing. The date and time of the webinar will be announced via email and through social media in the coming days.
ASHP will also provide FDA with comprehensive comments developed with member input by the comment deadline late this year. Additionally, it is important to note that the revised draft guidance is not currently enforceable. If your Board of Pharmacy attempts to enforce any element of the revised draft guidance, or if you have questions regarding the revised draft guidance, please contact Jillanne Schulte Wall.
